🧬 Beyond Boundaries: Exploring the Role of Extracellular Vesicles in Organ-Specific Metastasis in Solid Tumors
Context
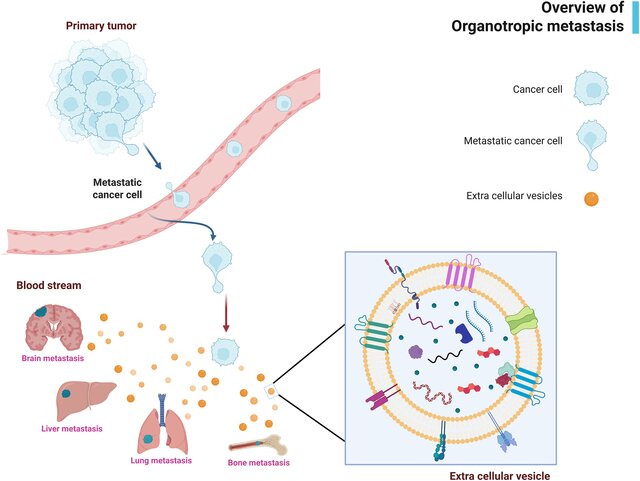
Metastasis remains the leading cause of cancer-related mortality in solid tumors. While classical models of metastasis have long focused on tumor cell–intrinsic properties, growing evidence suggests that successful metastatic colonization depends on active conditioning of distant organs before tumor cell arrival.
This comprehensive review examines the emerging role of tumor-derived extracellular vesicles (EVs) as key mediators of organ-specific (organotropic) metastasis. EVs function as long-range biological messengers, transporting proteins, lipids, and nucleic acids that reshape distant microenvironments and establish pre-metastatic niches favorable for future tumor growth.
Insight
A central concept emphasized in this review is that metastasis is not a random process, but a highly orchestrated, EV-guided event.
Tumor-derived EVs contribute to organotropism through several coordinated mechanisms:
- Selective EV cargo loading, including specific miRNAs, proteins, and integrins, which dictate interactions with target organs.
- Preferential EV uptake by resident cells in organs such as the lung, liver, brain, or bone, depending on EV surface signatures.
- Systemic reprogramming of distant tissues, often occurring well before circulating tumor cells are detectable.
This challenges the traditional “seed and soil” hypothesis by positioning EVs as active architects of the soil, rather than passive byproducts of tumor growth.
Mechanistic Perspective: How EVs Drive Organ-Specific Metastasis
1. Immune Remodeling
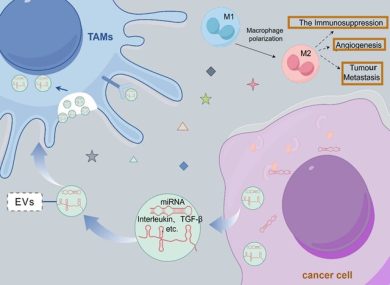
Tumor-derived EVs suppress anti-tumor immunity in target organs by:
- Inducing immunosuppressive phenotypes in macrophages and myeloid-derived suppressor cells,
- Inhibiting cytotoxic T cell and NK cell activity,
- Delivering immune checkpoint molecules (e.g., EV-associated PD-L1).
This immune conditioning creates a permissive environment for metastatic seeding.
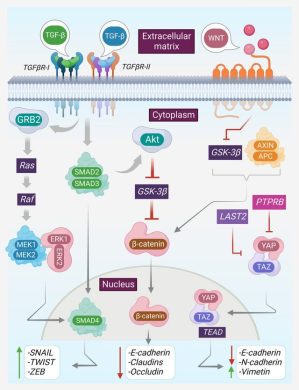
2. Vascular and Stromal Priming
EVs alter endothelial integrity and stromal behavior by:
- Increasing vascular permeability,
- Promoting angiogenesis,
- Activating fibroblasts toward cancer-associated phenotypes.
These changes facilitate tumor cell extravasation and survival in distant tissues.
3. Integrin-Mediated Organ Tropism
One of the most compelling findings discussed is the role of EV surface integrins in directing organ-specific accumulation:
- Certain integrin patterns preferentially guide EVs to the lung, liver, or brain,
- These integrins influence EV uptake and downstream signaling in resident cells,
- This mechanism provides a molecular explanation for organ-selective metastasis.
Clinical and Translational Implications
Understanding EV-mediated organotropism opens multiple translational avenues:
🔹 EVs as Predictive Biomarkers
Circulating EV profiles may:
- Predict metastatic organ preference,
- Enable earlier detection of metastatic risk,
- Support minimally invasive liquid biopsy approaches.
🔹 Therapeutic Targeting of EV Pathways
Potential strategies include:
- Inhibiting EV biogenesis or release from tumor cells,
- Blocking EV uptake in vulnerable organs,
- Neutralizing pro-metastatic EV cargo.
🔹 EV-Based Therapeutic Engineering
Conversely, EVs could be repurposed as:
- Targeted drug delivery vehicles,
- Immune-modulating nanocarriers,
- Precision tools for disrupting metastatic niches.
Challenges and Future Outlook
Despite their promise, several challenges remain:
- Technical limitations in EV isolation and characterization,
- Incomplete understanding of EV heterogeneity,
- Context-dependent and tumor-type-specific EV effects.
Future research must integrate systems biology, spatial profiling, and longitudinal patient data to translate EV-based insights into clinically actionable strategies.
Conclusion
This review reframes metastasis as a disease of dysregulated intercellular communication, with extracellular vesicles at its core. By crossing biological boundaries and programming distant organs, EVs enable tumors to extend their influence far beyond the primary site.
Targeting these communication networks may represent one of the most powerful strategies for preventing and treating metastatic disease in solid tumors.
Source
Beyond boundaries: exploring the role of extracellular vesicles in organ-specific metastasis in solid tumors
Frontiers in Immunology, 2025
DOI: 10.3389/fimmu.2025.1593834