🧬 Extracellular Vesicle-Based Drug Delivery Systems in Cancer Therapy:
A Comprehensive Deep-Dive into the Future of Precision Oncology
Context: Understanding the Rise of EVs in Cancer Drug Delivery
Over the past decade, extracellular vesicles (EVs), including exosomes (30–150 nm) and microvesicles (100–1000 nm), have emerged as one of the most biologically intelligent platforms for delivering therapeutic molecules directly to tumor cells.
Unlike synthetic nanoparticles (liposomes, polymeric NPs, dendrimers), EVs are:
- biocompatible,
- low-immunogenic,
- naturally optimized for intercellular communication,
- capable of crossing physical and biochemical barriers within the tumor microenvironment.
This review highlights how EVs efficiently transport diverse therapeutic cargo such as:
- chemotherapeutic agents (doxorubicin, paclitaxel),
- immunomodulatory proteins,
- siRNAs, miRNAs, and lncRNAs,
- CRISPR/Cas9 components,
- combination therapies targeting multiple pathways.
Their natural roles in cell–cell communication provide them with unique biological traits—traits that make EVs pre-adapted to function as precision drug carriers.
Insight: What Makes EVs Superior to Conventional Drug Delivery Systems?
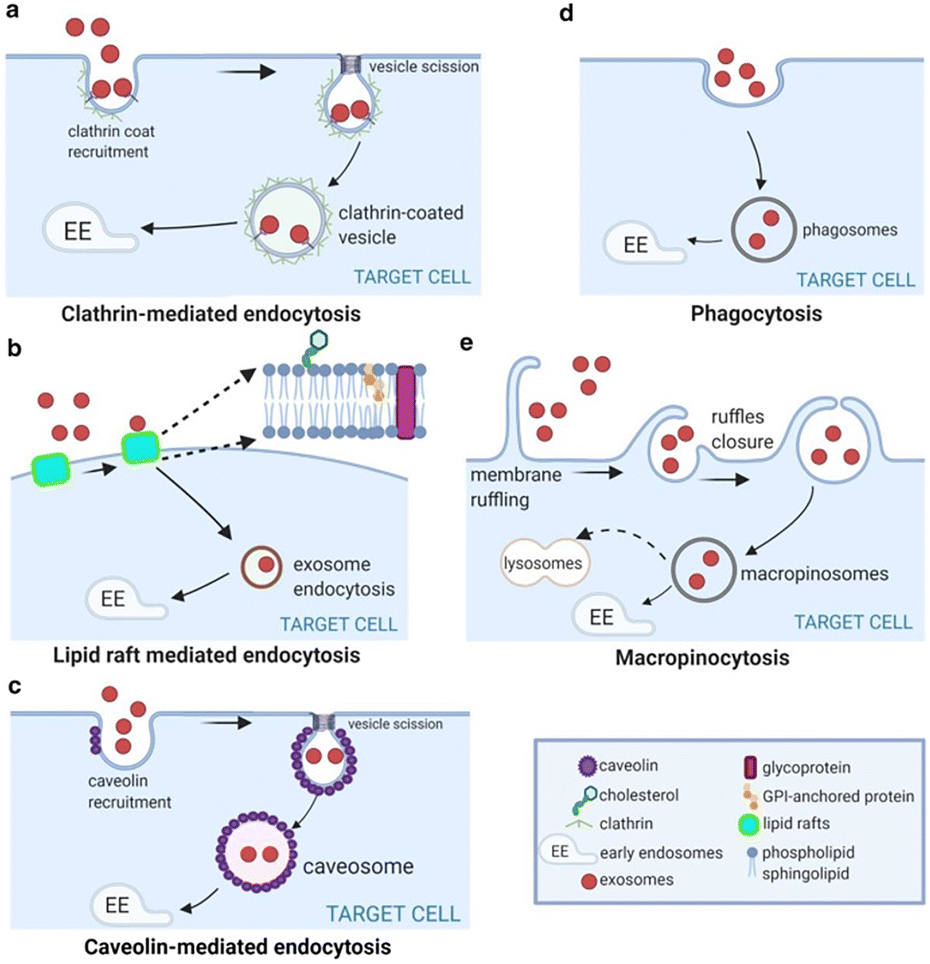
1. Tumor-Homing Ability Through Native Surface Molecules
EV membranes retain parent-cell proteins such as:
- integrins (αvβ3, α6β4),
- tetraspanins (CD9, CD63, CD81),
- adhesion molecules (ICAM-1, L1CAM),
- tumor-specific ligands.
These molecules enable EVs to recognize and fuse with tumor cells more efficiently than engineered nanoparticles.
2. Efficient Penetration of Dense Tumor Stroma
Solid tumors, especially pancreatic cancer, TNBC, and glioblastoma, are notoriously difficult to penetrate.
EVs overcome this barrier due to:
- their nanoscale size,
- deformable membrane structure,
- natural ability to migrate through hypoxic and fibrotic regions.
3. Protection of Therapeutic Cargo from Enzymatic Breakdown
The lipid bilayer provides a protective shell that shields:
- RNA molecules from RNases,
- proteins from proteases,
- small molecules from early degradation.
As a result, EV-delivered therapies show higher effective bioavailability.
4. Reduced Systemic Toxicity
Because EVs achieve enhanced delivery efficiency, therapeutic outcomes can be reached at much lower doses, minimizing adverse effects commonly associated with:
- chemotherapy
- immunotherapy
- siRNA therapy
5. Engineering Potential: Next-Generation EV Platforms
The field is moving rapidly toward designed EVs:
• Surface-engineered EVs
Displaying ligands (RGD peptides, antibodies, aptamers) for tumor-specific targeting.
• Hybrid EV–Lipid Nanoparticles
Combining the biological identity of EVs with the loading efficiency of synthetic systems.
• Stimuli-responsive EVs
Releasing cargo in response to:
- pH changes in tumors,
- redox conditions,
- enzyme-rich microenvironments.
• Gene-editing EVs
Delivering CRISPR/Cas machinery for precise genetic modifications.
Altogether, these innovations position EVs as sophisticated vehicles capable of reshaping modern cancer therapeutics.
Scientific Significance: Implications for Translational Oncology
The review underscores several transformative scientific directions:
1. Personalized Medicine
EVs inherit molecular signatures from their parent cells.
By engineering EVs from patient-specific cells (e.g., CAR-T cells, tumor-derived EVs), fully personalized therapies become feasible.
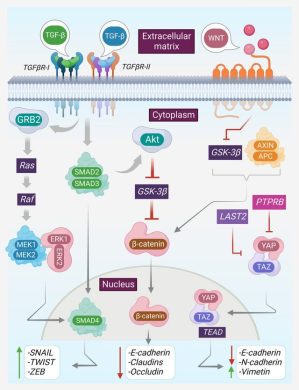
2. Combatting Chemoresistance
EVs can target:
- ABC transporter pathways,
- EMT-associated molecules,
- NF-κB and STAT3 signaling,
- stromal–tumor crosstalk.
This opens the door to reversing drug resistance via RNA-based EV therapies.
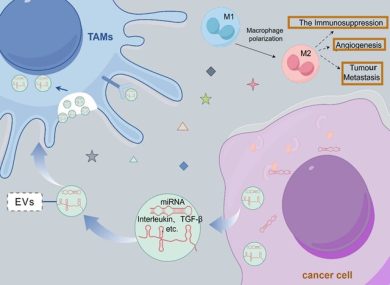
3. Immune Modulation
EVs can deliver:
- immunostimulatory cargo to activate T-cells,
- checkpoint-blocking molecules,
- siRNAs to suppress immunosuppressive macrophages.
This creates a synergy with existing immunotherapies.
4. Minimally Invasive Drug Administration
EVs can be delivered via:
- intravenous routes,
- local intratumoral injections,
- intranasal administration (promising for brain tumors),
- oral delivery (with appropriate encapsulation).
The potential for non-invasive delivery offers a major advantage in clinical practice.
5. Clinical Translation
Ongoing clinical trials show encouraging progress in using:
- MSC-derived EVs for RNA delivery
- tumor-derived EVs for personalized vaccines
- EV-based therapies for brain, ovarian, pancreatic, and colorectal cancers
As manufacturing and scaling technologies advance, EV-based therapeutics are poised to become cornerstone components of next-generation precision oncology.
Source
📄 Extracellular Vesicle-Based Drug Delivery Systems in Cancer Therapy
https://doi.org/10.3390/ijms26104835?urlappend=%3Futm_source%3Dresearchgate.net%26utm_medium%3Darticle