🧬 Extracellular Vesicles Derived from Breast Cancer Cells: Molecular Drivers of Tumor Progression, Metastasis, and Therapeutic Resistance
Context: A Comprehensive Look at Breast Cancer–Derived Extracellular Vesicles (EVs)
Breast cancer (BC) remains one of the most heterogeneous and dynamic malignancies, driven not only by genetic mutations within tumor cells but also by a complex network of intercellular communication within the tumor microenvironment (TME).
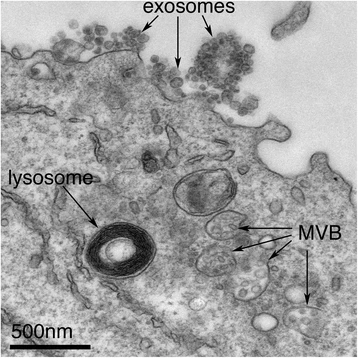
Among all communication mechanisms, extracellular vesicles (EVs) including exosomes and microvesicles stand out as highly efficient and selective carriers of oncogenic information.
The review examined in this analysis provides an extensive overview of how BC-derived EVs carry bioactive cargo such as:
- microRNAs (miRNAs),
- long non-coding RNAs (lncRNAs),
- circular RNAs (circRNAs),
- proteins and receptors,
- lipids,
- metabolites,
…all of which collectively influence tumor progression and metastatic behavior.
These vesicles do not randomly originate from breast cancer cells; rather, they are purposefully packaged with molecules that enhance tumor survival, plasticity, communication, and adaptability. Their role extends beyond local TME remodeling to pre-metastatic niche formation in organs such as bone, liver, and lung.
In essence:
Breast cancer EVs serve as mobile, functional extensions of tumor cells, redesigning the environment to favor malignancy.
Insight: How Breast Cancer EVs Act as Active Molecular Regulators
What makes EVs particularly remarkable is the level of strategic molecular engineering that cancer cells employ. The paper outlines several key mechanisms through which BC-derived EVs dynamically regulate malignancy:
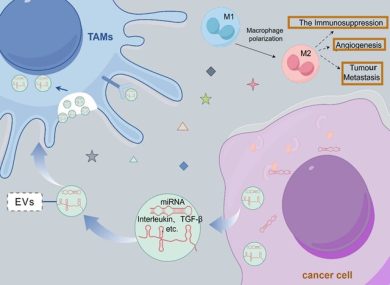
1. Angiogenesis and Vascular Remodeling
Breast cancer progression relies heavily on sustaining blood supply under hypoxic and stressful conditions. EVs enrich specific miRNAs—such as miR-210, miR-145, miR-130a—that promote:
- endothelial cell proliferation,
- increased vascular permeability,
- migration toward hypoxic tumor regions.
These angiogenic EV-miRNAs activate pathways like HIF-1α, VEGF, and Notch signaling.
This not only supports primary tumor growth but also facilitates metastatic extravasation.
2. Metastasis and Organ-Specific Colonization
One of the most compelling findings is how BC-derived EVs dictate where cancer spreads.
A notable example is EV-miR-9-5p, which modulates cholesterol metabolism in the liver, creating a hospitable niche for metastatic colonization.
Similarly:
- miR-181d-5p promotes lymph node metastasis,
- miR-105 disrupts endothelial barriers, enhancing intravasation,
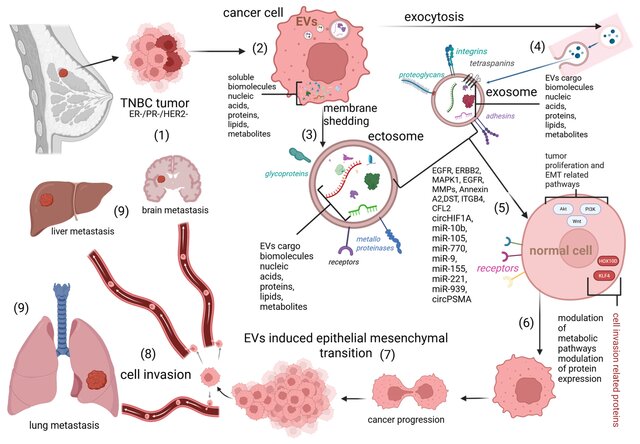
- and EV cargo in triple-negative breast cancer (TNBC) promotes aggressive dissemination.
These mechanisms illustrate that EVs act like pre-metastatic messengers, preparing distant tissues for tumor implantation long before cancer cells arrive.
3. EMT Induction and Invasive Phenotypes
The epithelial–mesenchymal transition (EMT) is a critical step for metastatic spread.
BC-derived EVs transport:
- miR-182-5p,
- miR-21,
- miR-200 family regulators,
- proteins activating CMTM7/EGFR/AKT signaling,
…all of which collectively suppress epithelial adhesion markers (like E-cadherin) and enhance invasive motility.
This EV-driven EMT generates stem-like cell properties, facilitating metastatic survival.
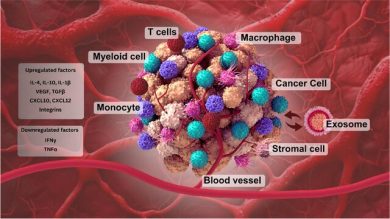
4. Immune Evasion and Immunomodulation
A tumor cannot progress without disabling the immune system. EVs derived from BC cells:
- polarize macrophages toward the M2 pro-tumor phenotype,
- suppress T-cell cytotoxicity,
- carry PD-L1–containing vesicles that inhibit immune recognition,
- modulate neutrophil and dendritic cell function toward immune suppression.
These modifications weaken anti-tumor immunity both locally and systemically.
5. Drug Resistance Mechanisms
Perhaps the most clinically significant aspect is EV-mediated drug resistance.
EVs containing miR-21, miR-155, miR-222/221, and proteins like Annexin A2:
- enhance survival signaling (PI3K/AKT, NF-κB),
- export chemotherapeutic drugs out of cells,
- transfer resistance traits to neighboring tumor cells,
- reprogram stromal cells into drug-protective niches.
This is especially prominent in TNBC, where EV signatures directly correlate with failure of chemotherapy.
6. Stromal and Adipocyte Reprogramming
Breast tissue is uniquely rich in adipocytes making up nearly 56% of its stromal composition.
Cancer-associated adipocytes (CAAs):
- release EVs enriched in miR-21,
- promote glycolytic and lipid metabolic rewiring in cancer cells,
- support drug tolerance and inflammation-driven tumor expansion.
The interplay between BC cells and CAAs via EVs is a powerful, underexplored mechanism shaping breast cancer biology.
Scientific Significance: Why Breast Cancer EVs Matter for Future Oncology
Breast cancer–derived EVs are not just byproducts they represent the next frontier for precision oncology.
This review highlights several impactful directions:
1. Liquid Biopsy and Early Detection
EVs circulating in blood, saliva, or breast ductal fluid contain stable RNA markers that can:
- detect cancer earlier,
- predict tumor subtype,
- monitor treatment response,
- track recurrence or metastasis.
EV-miRNAs and EV-lncRNAs offer greater stability than cell-free RNA.
2. Personalized Therapy Based on EV Signatures
Each breast cancer subtype ER+, HER2+, TNBC releases a unique EV profile.
These EV fingerprints can guide:
- therapy selection,
- targeted drug design,
- precision stratification of high-risk patients.
3. Engineered EVs as Therapeutic Vehicles
Due to their natural biocompatibility and targeting capabilities, EVs can be engineered to deliver:
- tumor-suppressor miRNAs,
- siRNAs or CRISPR components,
- small-molecule drugs,
- immunomodulators.
This approach promises lower toxicity and higher specificity.
4. Targeting EV Release and Uptake
Blocking EV biogenesis (e.g., GW4869, Rab inhibitors) or interfering with EV uptake pathways offers potential to:
- disrupt metastasis,
- reduce drug resistance,
- weaken tumor adaptability.
5. Clinical Outlook
Hundreds of ongoing clinical trials are exploring miRNA biomarkers, with a growing shift toward EV-based diagnostics.
For breast cancer specifically, EV-miRNAs show significant potential to:
- revolutionize early detection,
- refine patient stratification,
- and enhance targeted therapies in TNBC and metastatic disease.
Conclusion
Extracellular vesicles derived from breast cancer cells are not passive waste carriers
they are active orchestrators of tumor evolution, shaping every stage from local growth to metastatic invasion.
Understanding and harnessing EV-mediated communication is likely to become a cornerstone of next-generation therapies and precision oncology, influencing both diagnostics and clinical interventions.
Source
📄 Extracellular Vesicles Derived from Breast Cancer Cells: Emerging Biomarkers of Tumor Progression and Metastasis
DOI:10.3390/biom15081195