🧬 Tumor-Derived Small Extracellular Vesicles (TD-sEVs):
Comprehensive Mechanisms in Cancer Progression, Prediction, and Therapy
Context
Tumor-derived small extracellular vesicles (TD-sEVs) have emerged as central mediators of cancer progression.
Released by primary and metastatic tumor cells, these nanosized vesicles (typically <200 nm) carry a rich and biologically active cargoin, cluding proteins, miRNAs, lncRNAs, circRNAs, lipids, and metabolites, that mirrors the molecular profile of the tumor.
This review emphasizes that TD-sEVs are not passive waste products, but highly functional messengers that orchestrate tumor–host interactions, remodel the tumor microenvironment, and facilitate local and distant cancer progression.
Because TD-sEVs circulate in blood, lymph, urine, saliva, ascites, and other body fluids, they have become attractive tools for early diagnosis, liquid biopsy, and therapy monitoring.
Insight
What makes TD-sEVs uniquely powerful is their ability to influence multiple hallmarks of cancer simultaneously. The review highlights several mechanistic layers:
1. Drivers of Tumor Growth and Survival
TD-sEVs deliver oncogenic molecules, such as miR-21, miR-155, miR-210, TGF-β, and various growth factors, that activate pathways including:
- PI3K/AKT
- STAT3
- MAPK/ERK
- NF-κB
These signals enhance cell proliferation, inhibit apoptosis, and sustain long-term tumor survival.
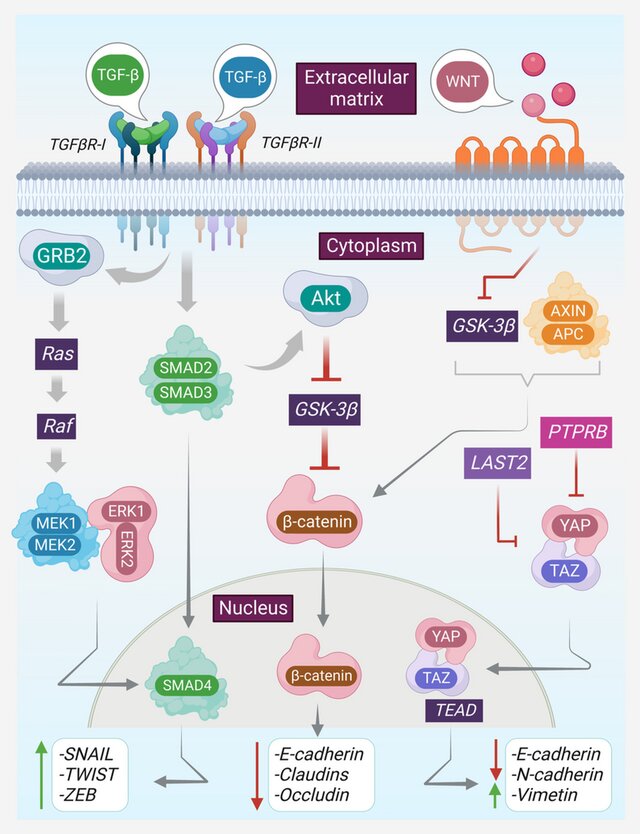
2. Enhancers of Invasion, EMT, and Metastasis
TD-sEVs play a pivotal role in:
• Inducing EMT (Epithelial–Mesenchymal Transition)
Cargo such as miR-200 family repressors, SNAIL/TWIST regulators, and lncRNAs drive:
- loss of E-cadherin
- gain of Vimentin
- increased motility and invasiveness
• ECM Remodeling
TD-sEVs transport matrix-modifying enzymes such as MMPs, enabling tumor cells to invade deeper tissues.
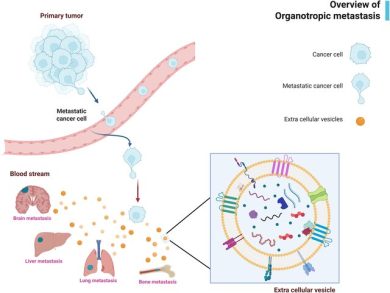
• Pre-Metastatic Niche Formation
Before tumor cells arrive, TD-sEVs prepare distant organs by:
- altering stromal cells
- increasing vascular permeability
- promoting inflammation
- suppressing local immune responses
- reorganizing extracellular matrix structure
This creates a fertile “soil” for metastatic colonization.
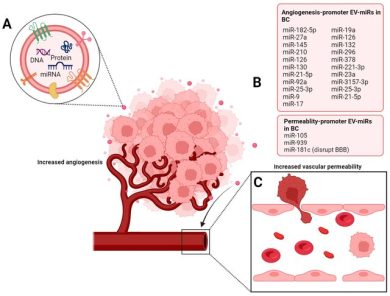
3. Angiogenesis and Vascular Adaptation
TD-sEVs contribute to tumor vascularization by transporting:
- VEGF-related molecules
- pro-angiogenic miRNAs
- endothelial-activating proteins
These vesicles stimulate endothelial migration, tube formation, and permeability, enabling tumors to develop their own supportive blood supply.
4. Immune Suppression and Immune Escape
The review highlights multiple immunomodulatory roles of TD-sEVs:
- Delivery of PD-L1 to inhibit T-cell cytotoxicity
- Transfer of immunosuppressive cytokines such as TGF-β
- Reprogramming macrophages toward M2 phenotypes
- Suppressing dendritic cell maturation
- Modulating NK-cell activity
This immune rewiring allows tumors to grow and metastasize with minimal immune resistance.
5. Contributors to Drug Resistance and Therapy Failure
One of the most clinically relevant insights is the role of TD-sEVs in therapy escape.
They facilitate resistance by:
- exporting chemotherapy drugs
- delivering anti-apoptotic miRNAs
- enhancing DNA repair pathways
- activating oxidative stress–response mechanisms
- transferring resistant phenotypes to neighboring cells
These mechanisms significantly reduce the effectiveness of chemotherapy, targeted therapy, and even immunotherapy.
6. TD-sEV microRNAs as Functional Oncogenic Mediators
The review deeply emphasizes the dual nature of sEV-miRNAs:
Pro-tumor miRNAs (oncogenic):
- enhance EMT
- promote angiogenesis
- induce immune escape
- stimulate invasion
- drive metastasis
Anti-tumor miRNAs (tumor-suppressive):
- inhibit proliferation
- reverse EMT
- suppress angiogenesis
- modulate stromal remodeling
This duality makes sEV-miRNAs both diagnostic markers and therapeutic targets.
Scientific Significance
1. Liquid Biopsy and Non-Invasive Diagnostics
Because TD-sEVs circulate systemically and contain stable cargo protected by lipid membranes, they serve as exceptional biomarkers for:
- early detection
- tumor subtype classification
- metastasis prediction
- recurrence monitoring
- therapy response assessment
Their stability, specificity, and accessibility make them superior to free-circulating RNA markers.
2. Predictive Indicators for Therapy Response
TD-sEV cargo can forecast sensitivity or resistance to:
- chemotherapy
- radiotherapy
- immunotherapy
- targeted therapy
This can guide personalized treatment plans and help avoid ineffective regimens.
3. Therapeutic Opportunities: Targeting EV Pathways
The review discusses several strategies:
• Blocking EV biogenesis
Inhibiting pathways like:
- Rab GTPases
- ESCRT complexes
- neutral sphingomyelinase
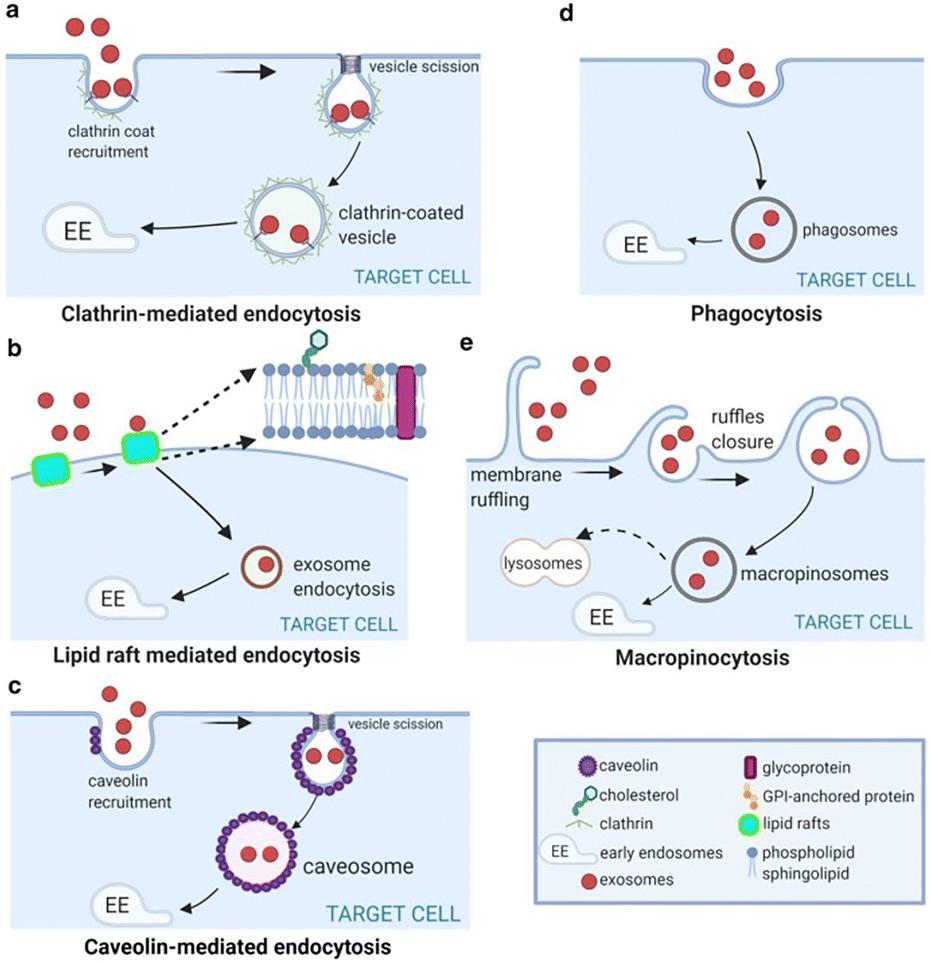
• Inhibiting EV uptake
Preventing tumor or stromal cells from internalizing pro-metastatic vesicles.
• Engineering therapeutic EVs
Loading EVs with:
- tumor-suppressive miRNAs
- siRNAs
- immunomodulators
- chemotherapeutics
This approach promises high specificity and reduced toxicity.
Conclusion
Tumor-derived sEVs are now recognized as master regulators of cancer progression, capable of reshaping every stage of tumor evolution, from early invasion to metastatic colonization, immune escape, and treatment failure.
Their roles in communication, biomarker discovery, and therapeutic development make them central to the future of precision oncology.
As research advances, TD-sEVs are expected to become foundational tools for:
- early diagnosis
- metastasis prediction
- personalized therapy design
- next-generation EV-based treatments
TD-sEVs represent both a challenge and an opportunity, an intricate communication system that fuels cancer, and simultaneously a powerful resource for defeating it.
Source
📄 Tumor-derived small extracellular vesicles in cancer invasion and metastasis: molecular mechanisms, and clinical significance