🧬 Caveolin-1 in Tumor-Derived Extracellular Vesicles:
Context
Caveolin-1 (Cav-1) is a scaffolding protein located in caveolae, specialized lipid-raft microdomains involved in membrane trafficking, mechanotransduction, and signal modulation.
Recent discoveries reveal that Cav-1 is frequently incorporated into tumor-derived extracellular vesicles (TD-EVs), where it functions as an active regulator of intercellular communication rather than a passive structural molecule.
The reviewed study highlights how Cav-1-positive EVs contribute to nearly every stage of cancer progression.
Through the transfer of Cav-1 and associated signaling components, TD-EVs reshape the tumor microenvironment (TME), influence stromal dynamics, and prepare metastatic niches.
As evidence grows, Cav-1 is emerging as a key molecular determinant in understanding cancer aggressiveness, metastatic behavior, and therapeutic failure.
Insight
What distinguishes this review is its comprehensive demonstration that Cav-1 acts as a signaling amplifier carried by EVs, enabling tumors to manipulate distant and neighboring cells with high precision.
Below is a detailed breakdown of mechanistic insights described in the review:
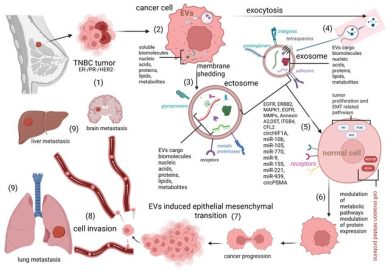
1. Cav-1-EVs Drive EMT and Cell Motility
Cav-1 packaged into EVs activates motility-associated pathways such as:
- Src/FAK signaling → cytoskeletal remodeling
- PI3K/AKT activation → survival and migration
- β-catenin signaling → EMT initiation
By reducing epithelial adhesion molecules (e.g., E-cadherin) and enhancing mesenchymal markers (e.g., vimentin), Cav-1-EVs directly induce epithelial–mesenchymal transition, a critical prerequisite for metastasis.
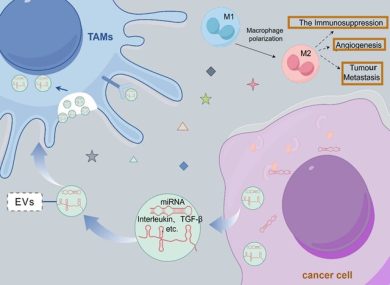
2. Fibroblast Reprogramming and CAF Phenotype Induction
Cav-1-positive EVs reprogram normal fibroblasts into cancer-associated fibroblasts (CAFs).
These CAFs exhibit:
- increased α-SMA expression
- secretion of pro-invasive cytokines
- ECM remodeling via MMPs
- promotion of angiogenesis
This process creates a supportive stromal environment that accelerates tumor invasion and metastatic preparation.
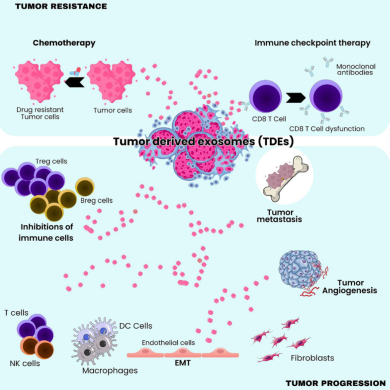
3. Angiogenesis and Vascular Remodeling
The review highlights that Cav-1-EVs enhance endothelial cell activity by:
- increasing vascular permeability
- promoting endothelial tube formation
- stimulating VEGF-associated pathways
These changes facilitate intravasation of tumor cells and lay the groundwork for pre-metastatic niche development in distant organs.
4. Metabolic Reprogramming via EV-Mediated Cav-1 Transfer
Cav-1 within EVs alters lipid transport, caveolae-dependent endocytosis, and mitochondrial arrangement.
This enhances tumor cell survival under:
- hypoxia
- oxidative stress
- nutrient deprivation
Metabolic plasticity is a hallmark of metastatic potential, and Cav-1-EVs directly support this adaptation.
5. Drug Resistance and Therapy Failure
One of the most clinically relevant observations is that Cav-1-positive EVs contribute to intercellular transfer of drug-resistant traits.
They modulate:
- apoptotic threshold
- drug efflux pump expression
- DNA repair pathways
- pro-survival signaling cascades
This allows not only the donor tumor cells but also surrounding cells to become less responsive to treatment.
Scientific Significance
The review underscores several clinically meaningful opportunities for diagnostics and therapy:
1. Cav-1-EVs as Biomarkers
Cav-1 levels in circulating EVs correlate with:
- tumor stage
- metastatic burden
- invasive phenotypes
- response to therapy
Because EV cargo is stable and protected by lipid membranes, Cav-1-EVs represent a new class of robust, minimally invasive biomarkers.
2. Predicting Therapy Response
Cav-1-EV profiles may predict:
- resistance to chemotherapy
- reduced response to radiotherapy
- escape from targeted inhibitors
- immunotherapy non-responsiveness
This positions Cav-1-EVs as valuable companion diagnostics for treatment planning.
3. Therapeutic Targeting of Cav-1-EV Pathways
Potential strategies include:
- inhibiting Cav-1 packaging into EVs
- blocking EV release (e.g., via ESCRT/Rab pathway modulation)
- preventing EV uptake by stromal or endothelial cells
- designing Cav-1-deficient engineered EVs to safely deliver drugs without activating invasion pathways
These approaches could significantly reduce tumor spread and overcome resistance mechanisms.
4. Integration into Precision Oncology
Because Cav-1 influences mechanics, metabolism, angiogenesis, and cell migration simultaneously, it represents a multi-axis target with potential to improve:
- metastasis prevention
- early diagnosis
- stromal-targeted therapies
- EV-based nanomedicine
Conclusion
Caveolin-1 in tumor-derived extracellular vesicles is more than a structural scaffold—it is a master regulator of tumor communication, enabling cancer cells to sculpt their microenvironment, manipulate stromal partners, promote vascular adaptation, and resist therapy.
As research advances, Cav-1-EVs are poised to become:
- key liquid biopsy markers,
- therapeutic targets, and
- foundational components of next-generation EV-based drug delivery systems.
Understanding Cav-1-mediated EV signaling is therefore essential for developing effective metastasis-blocking and precision oncology strategies.
Source
📄 The role of Caveolin-1 in tumor-derived extracellular vesicle biology and its implications
https://doi.org/10.3389/fcell.2025.1656953