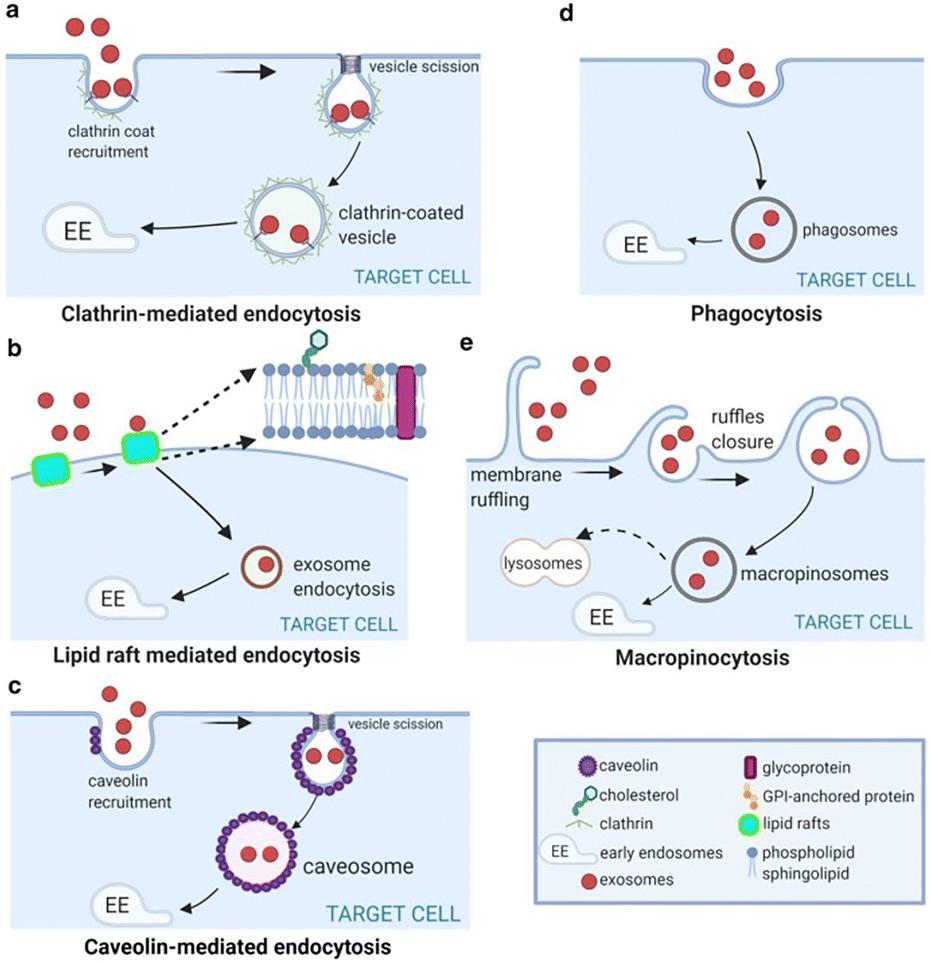
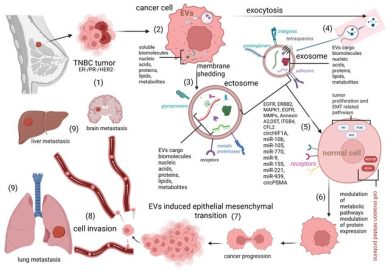
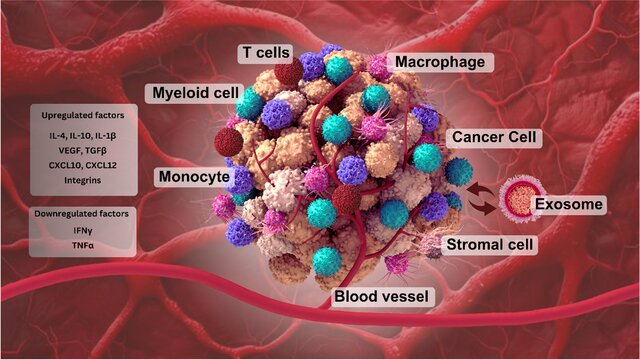
Exosomal miRNAs: The Tumor’s Trojan Horse in Selective Metastatic Organotropism
Context
Metastasis remains the leading cause of cancer-related mortality, yet it is increasingly clear that metastatic spread is not a stochastic event. Instead, many tumors display a striking preference for colonizing specific organs, a phenomenon known as selective metastatic organotropism.
Recent advances in extracellular vesicle biology have revealed that tumor-derived exosomal microRNAs (exo-miRNAs) play a pivotal role in orchestrating this selectivity. Exosomes act as long-range communication vehicles, transporting miRNAs from primary tumors to distant organs, where they modify local cellular and molecular landscapes before the arrival of circulating tumor cells.
This review conceptualizes exosomal miRNAs as a biological “Trojan horse”, molecular agents that covertly infiltrate distant tissues, reprogram resident cells, and create permissive environments for future metastatic colonization.
Insight
What distinguishes exosomal miRNAs from other soluble tumor-derived factors is their context-dependent specificity. Tumor cells do not release random miRNA cargo; rather, they selectively package miRNAs that align with the biological vulnerabilities of particular target organs.
1. Exosomal miRNAs as Molecular Directors of Organotropism
Distinct cancers exhibit unique exosomal miRNA signatures that correlate strongly with their preferred metastatic destinations:
- miR-21 and miR-210 promote lung metastasis by activating inflammatory signaling, angiogenesis, and endothelial permeability.
- miR-122 facilitates liver metastasis by reprogramming glucose uptake and lipid metabolism in hepatocytes, effectively creating a nutrient-rich niche for tumor cells.
- miR-181c disrupts tight junction proteins in endothelial cells, weakening the blood–brain barrier and enabling brain metastasis.
These miRNAs alter stromal and vascular cell behavior in a manner that mirrors the metabolic and structural demands of metastatic growth.
2. Pre-Metastatic Niche Formation
Exosomal miRNAs actively remodel distant tissues by:
- converting fibroblasts into pro-tumorigenic phenotypes,
- increasing vascular leakiness to facilitate tumor cell extravasation,
- polarizing macrophages toward immunosuppressive M2 states,
- reorganizing extracellular matrix composition and stiffness.
Through these coordinated changes, target organs are transformed into biologically receptive “soil” well before tumor cells disseminate.
3. Immune Reprogramming and Metastatic Escape
A critical aspect of exo-miRNA function lies in immune modulation. Tumor-derived exosomal miRNAs suppress immune surveillance by:
- inhibiting T-cell activation and proliferation,
- reducing NK cell cytotoxicity,
- impairing antigen presentation pathways.
This immune reprogramming creates a window of opportunity for circulating tumor cells to survive, seed, and expand within secondary organs.
4. Dynamic Regulation and Tumor Plasticity
Importantly, exosomal miRNA cargo is not static. Hypoxia, therapeutic pressure, and tumor evolution dynamically reshape miRNA composition, allowing tumors to adapt their metastatic strategies over time. This plasticity underscores the complexity, and therapeutic challenge, of targeting exosomal miRNA signaling.
Scientific Significance
The biological insights reviewed here open several clinically transformative avenues:
1. Exosomal miRNAs as Liquid Biopsy Biomarkers
Circulating exosomal miRNA profiles offer minimally invasive tools to predict:
- future metastatic organ involvement,
- disease progression trajectories,
- likelihood of recurrence,
- and therapeutic responsiveness.
Their stability within vesicles makes them particularly attractive candidates for clinical implementation.
2. Anti-Metastatic Therapeutic Strategies
Potential intervention points include:
- inhibiting exosome biogenesis or release,
- blocking uptake of miRNA-loaded exosomes by target organs,
- selectively neutralizing pro-metastatic miRNAs,
- delivering engineered anti-metastatic miRNAs via therapeutic extracellular vesicles.
Such strategies aim not merely to kill tumor cells, but to interrupt metastatic communication networks.
3. Precision Oncology Applications
Each tumor type encodes a distinct exosomal miRNA “metastasis signature.” Decoding these signatures may enable personalized prediction, monitoring, and prevention of metastatic spread, addressing one of the most critical unmet needs in oncology.
Conclusion
Exosomal miRNAs represent one of the most sophisticated mechanisms by which tumors orchestrate metastasis. Acting as molecular Trojan horses, they silently remodel distant tissues, suppress immune defenses, and dictate organ-specific colonization.
Targeting exosomal miRNA signaling offers a paradigm shift, from treating metastasis after it occurs to preventing metastatic competence before dissemination.
Source
📄Exogenous Extracellular Vesicles as Emerging Platforms in Translational Medicine
https://doi.org/10.15212/bioi-2025-0122